REST-dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors
Description
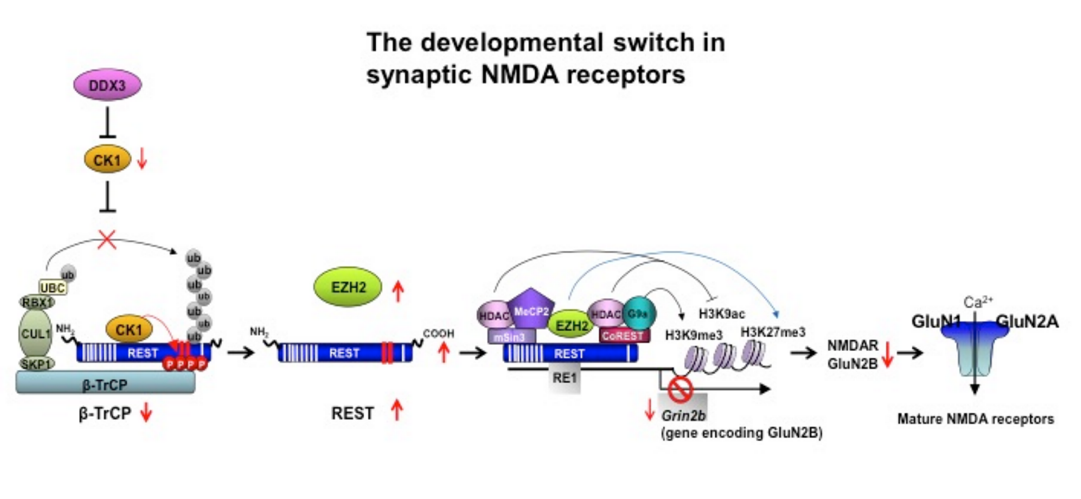
NMDA receptors (NMDARs) are critical to synaptogenesis, neural circuitry and higher cognitive functions. A hallmark feature of NMDARs is an early postnatal developmental switch in receptor subunit composition from primarily GluN2B- to primarily GluN2A-containing receptors. This is significant in that GluN2B expression can restrict synaptic incorporation of AMPA receptors, reduce the threshold for and enhance the magnitude of long-term potentiation, and promote hippocampus-dependent learning, plasticity-induced spine growth and dendritic patterning critical to information processing. Although the switch in phenotype has been an area of intense interest for nearly two decades, the mechanisms that trigger it and the link between experience and the switch in NMDARs are unclear. We recently discovered a novel role for the transcriptional repressor REST in the developmental switch in synaptic NMDARs. We found that REST is activated at a critical window of time during postnatal development and acts via epigenetic remodeling to repress GluN2B expression and alter NMDAR properties at rat hippocampal synapses. Acute knockdown of REST by direct delivery of shRNA into the hippocampus of intact rats prevents the decline in GluN2B and switch in NMDAR phenotype. These findings establish a causal relation between REST-dependent epigenetic remodeling and the switch in NMDARs at hippocampal synapses. We further show that adverse experience in early life in the form of maternal deprivation disrupts activation of REST and acquisition of the mature NMDAR phenotype. More recently, we found that REST is regulated at the level of protein stability via β-TrCP-dependent ubiquitin-based proteasomal degradation in differentiated neurons and identified casein kinase 1 (CK1) as an upstream signal that negatively regulates REST in neurons under physiological conditions. These findings document that activation of REST plays a pivotal role in experience-dependent fine-tuning of genes involved in synaptic plasticity and enhance our understanding of the control of NMDAR function as it pertains to memory, synaptic stabilization, brain development and cognitive information flow.
Speaker Bio
R. Suzanne Zukin obtained her undergraduate degree from Bryn Mawr College cum laude with Honors in Chemistry and her PhD degree with Distinction from the Johns Hopkins University School of Medicine. She performed her postdoctoral training with Daniel E. Koshland, Jr. at the University of California at Berkeley, where she made the discovery that bacteria such as Salmonella can use chemoreceptors to detect metal ions such as Mg2+. This work was published in the journal Science. In 1977 Suzanne assumed the position of Assistant Professor at the Albert Einstein College of Medicine. Within 2 years, she established a large, vibrantly active laboratory, was awarded several grants from the NIH including the prestigious Research Career Development Award, an NSF grant and an American Heart Young Investigators grant. In 1979 Suzanne published her seminal discovery of the phencyclidine receptor, which mediates psychotomimetic actions of phencyclidine, ketamine and sigma opiates and showed it was a site within the NMDA receptor channel. The paper reporting this work became a citation classic (1992).
Since 1987 Suzanne has been Professor of Neuroscience and Director of the Neuropsychopharmacology Center at the Albert Einstein College of Medicine. In 2008, she became the first F.M. Kirby Professor in Neural Repair and Protection. Her research interests address the regulation of glutamate receptor gene expression, local protein synthesis, trafficking and gating, and mechanisms underlying ischemia-induced neuronal death. She is perhaps best known for her work reporting the cloning of functionally-distinct NMDA receptor splice variants, regulation of NMDA receptor trafficking by PKC, and regulation of NMDA receptor Ca2+ permeability and Ca2+ signaling in spines by PKA and neurotransmitters that signal through PKA. In 2012 Zukin published her seminal finding that the gene silencing transcription factor REST orchestrates epigenetic remodeling and drives the switch in synaptic NMDA receptors during brain development. In addition, she is known for her landmark discovery that Ca2+-permeable AMPA receptors are expressed in response to neuronal activity and insults and play a critical role in neuronal injury and neuronal death. It is now well established that Ca2+-permeable AMPA receptors are important in synaptic plasticity, the response to stress and a myriad of brain disorders including stroke, global ischemia, epilepsy, spinal cord injury, ALS, cocaine addiction and withdrawal, and alcoholism. Zukin further showed that the mechanism underling the switch in AMPAR phenotype involves REST-dependent epigenetic remodeling. In addition to her research activities, Suzanne has served as a standing member of numerous Study Sections and on the editorial board of Journal of Biological Chemistry, Molecular Pharmacology, Journal of Neuroscience, Molecular Brain Research, Neurobiology of Disease (Review Editor), Neuropsychopharmacology, Synapse, Frontiers in Neuroscience (Associate Editor) and, most recently,European Journal of Neurodegenerative Diseases, and Genetics and Epigenetics. In 1983 she was inducted into the prestigious American College of Neuropsychopharmacology and in 2005 was elected a Lifetime Fellow. In 2001 she presented the George H. Bishop Lecture at Washington University, in 2009, the Spivack Distinguished Lecture at Boston University, and in 2013, the Swammerdam Lecture at the University of Amsterdam. In 2009, she was a recipient of the prestigious McKnight Neuroscience of Brain Disorders Award and in 2014, the NARSAD Distinguished Investigator Award. As a testimony to her leadership in two different fields, Suzanne chaired the Gordon Research Conference on Opiate Receptors in 1987, and in 2015, the Gordon Research Conference on Excitatory Synapses. She has published 185 peer-reviewed papers in eminent journals including Science, Nature Neuroscience, Neuron, Molecular Cell, Developmental Cell, and Journal of Neuroscience and 50 book chapters, and edited a Special Topics issue of Frontiers in Molecular Neuroscience.

