Optimal long-term memory in complex synapses with bounded dynamical variables
Description
**Faculty Candidate - Computational Neuroscience & Cognition**
Memory consolidation at the level of synaptic connections
relies on a complex network of highly diverse biochemical processes
that operate on a wide range of different timescales. Identifying
their computational roles and understanding how these intricate
networks of interactions support synaptic memory formation and
maintenance requires an appropriate theoretical framework.
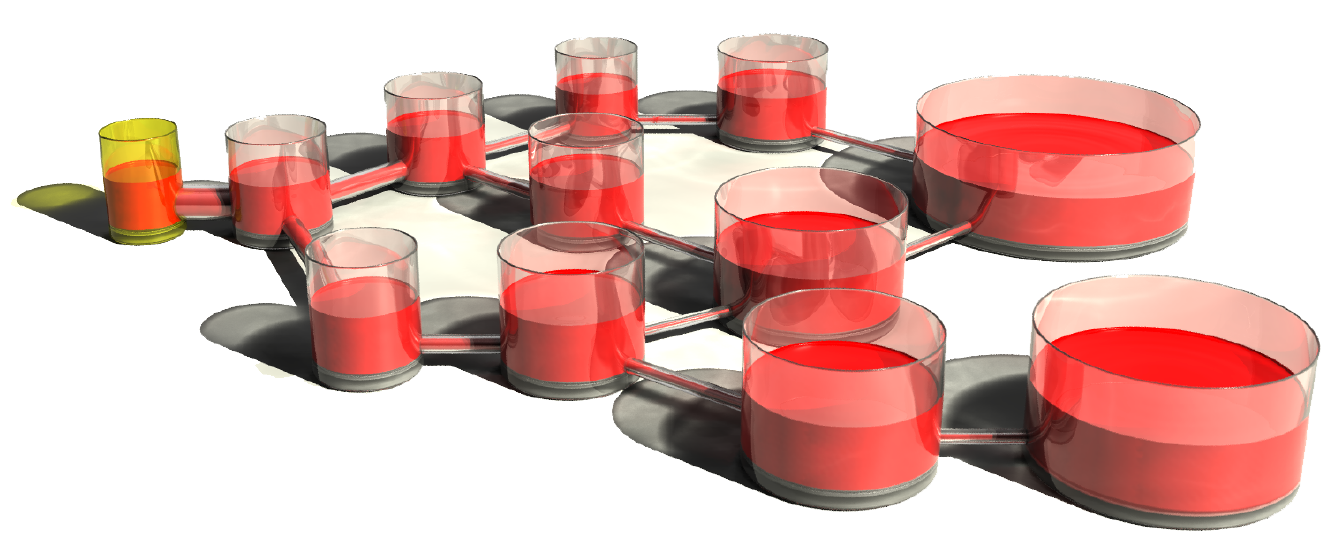
In this work we construct a broad class of synaptic models with
tightly bounded dynamical variables and efficacies that can
efficiently harness biological complexity to store and preserve
numerous memories. The number of storable memories grows almost
linearly with the number of synapses, which constitutes a substantial
improvement over the square root scaling of previous models,
especially when large neural systems are considered. In addition, the
initial memory strength is also high in these models, and scales
approximately like the square root of the number of synapses.
These favorable properties are achieved by combining together multiple
dynamical processes that operate on different timescales, to ensure
the memory strength decays as slowly as the inverse square root of the
age of the corresponding synaptic modification. This decay implements
an optimal compromise between large memory strengths and long lifetimes,
maximizing the area under the signal to noise ratio curve. Memories are
initially stored in fast variables and then progressively transferred
to slower variables. Importantly, in our case the interactions between
fast and slow variables are bidirectional, in contrast to the
unidirectional cascades of previous models. Each synapse only requires
a small number of variables that can have very limited precision.
The proposed models are robust to perturbations of parameters and can
capture several properties of biological memories, which include
delayed expression of synaptic potentiation and depression, synaptic
metaplasticity, and spacing effects. We discuss predictions for the
autocorrelation function of the synaptic efficacy that can be tested
in plasticity experiments involving long, balanced sequences of
synaptic modifications

