Study finds a new culprit for epileptic seizures
by
Anne Trafton, MIT News Office
Image
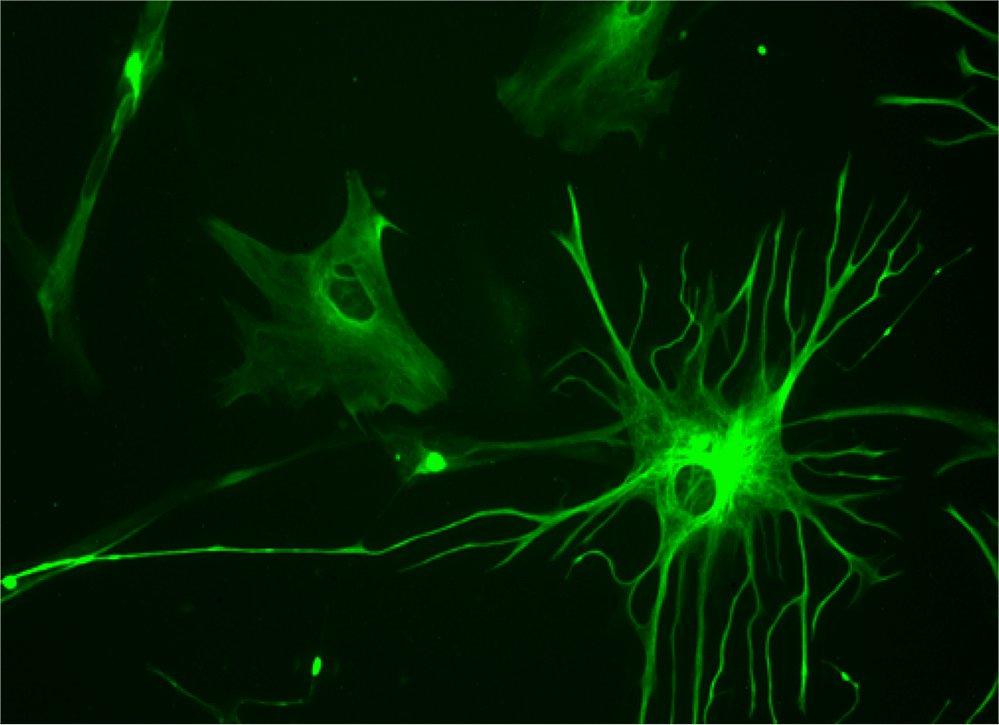
Neuroscientists have found that glial cells, which make up about half the cells in the brain, may play more than just a supporting role.
Epileptic seizures occur when neurons in the brain become excessively active. However, a new study from MIT neuroscientists suggests that some seizures may originate in non-neuronal cells known as glia, which were long believed to play a mere supporting role in brain function.
In a study of fruit flies, the researchers identified a glial-cell mutation that makes the flies much more prone to epileptic seizures. Mutations in the gene, which influences glial cells’ communication with neurons, appear to make neurons much more excitable. That excitability makes the flies more likely to seize in response to environmental stimuli, such as extreme temperatures.
This is the first time anyone has shown, in living animals, that mutations in glial cells can produce epileptic seizures. Counteracting the effects of the glial mutation may be a promising new strategy for developing epilepsy treatments, says Troy Littleton, an MIT professor of biology and leader of the research team.
“This signaling pathway from glia to neurons likely controls the firing properties of neurons, and if it’s hyperactivated, the neurons fire too much. Anything that would prevent that hyperactivity might be a potential therapeutic,” says Littleton, who is also a member of MIT’s Picower Institute for Learning and Memory.
Littleton and lead author Jan Melom, a graduate student in MIT’s Department of Biology, described their new findings in the Jan. 16 online edition of the Journal for Neuroscience.
Origin of seizures
Glial cells, which make up about half of the cells in the brain, have many supporting roles, including cushioning neurons and helping them form connections with one another. However, in recent years, neuroscientists have found evidence of more important functions, such as assisting in communication between neurons.
Previous studies had shown that glial cells become hyperactive during epileptic seizures, but it wasn’t clear whether the glial cells’ hyperactivity was causing the seizure or if they were merely reacting to neuronal hyperactivity. The new study shows that the glial cells are indeed provoking the seizure, according to the researchers.
“We know that we’re knocking out a gene that’s only expressed in this one cell population, of glia,” Melom says. “It’s unambiguous that the seizure originally arises from defects in the glia rather than in the neurons.”
Littleton’s lab uses fruit flies as a model organism for studies of neuronal development and the formation of synapses, the connections between neurons. To pinpoint the genes involved in these processes, they mutate fruit fly genomes and screen the resulting flies for abnormal traits. Most of the mutations occur in neurons, but in this case, the researchers found that flies with glial mutations in a gene they named zydeco caused the flies to undergo seizures at high temperatures.
Further study of the zydeco gene revealed that its normal function is to get rid of calcium inside the glia. This helps to regulate glial fluctuations in calcium levels, which the MIT team observed for the first time in fly glia.
When zydeco is mutated, the fluctuations stop, causing calcium to build up inside the glial cells. Through an unknown process, this induces nearby neurons to become hyperactive and makes them more likely to seize in response to heat, cold or shaking. “It’s clear that their nervous systems are hyperexcitable to a lot of environmental stimuli,” Littleton says.
This is one of the few examples scientists have found of glia activity influencing neurons’ firing.
“Neurons are still carrying the main information,” Littleton says. “They’re the ones talking. But glia are somehow listening in and modulating the neurons’ output. If that output is disrupted by glia, it causes the whole system to fail.”
Sources of excitation
The researchers are now exploring the link between glial calcium levels and neuronal excitability. One theory is that too much calcium produces a flood of neurotransmitters or other factors that glia normally secrete in a tightly regulated fashion. This unregulated secretion could hyperexcite the neurons, Littleton says.
“Glia are enlisted into the conversation that neurons are having, and they may modulate that by releasing these factors that control activity,” Littleton says. “Elevated calcium may create a situation where the glia are no longer releasing these factors in a regulated way, but they’re releasing them all the time.”
In the human brain, astrocytes are the cells most similar to the fruit fly glial cells observed in this study, according to the researchers. These cells envelop the cell bodies of neurons and are also the most abundant type of glial cell in the human brain. It is not yet known whether these cells express one of the five human versions of the zydeco gene.
Although further study is needed, the findings suggest that preventing calcium from building up in glial cells or astrocytes could reduce neuronal excitability and decrease the tendency to seize in response to environmental stimuli, according to the researchers. “It’s still a long way off, but the fly work suggests that reducing glial calcium levels would be effective in toning down excitability,” Littleton says.
Such drugs might also eliminate the sedative side effects often seen with current epilepsy medications, says Maiken Nedergaard, a professor of neurosurgery at the University of Rochester who was not involved in the research.
“You could envision new medications that target glial cells and don’t slow down the brain as much,” Nedergaard says, adding that the study is a major step forward in understanding glial cells’ role in the brain.
The MIT researchers are now looking for other components of the pathway that connects glial calcium levels to neuronal hyperactivity, which could lead to the discovery of other potential drug targets.
The study was funded by the National Institutes of Health.
In a study of fruit flies, the researchers identified a glial-cell mutation that makes the flies much more prone to epileptic seizures. Mutations in the gene, which influences glial cells’ communication with neurons, appear to make neurons much more excitable. That excitability makes the flies more likely to seize in response to environmental stimuli, such as extreme temperatures.
This is the first time anyone has shown, in living animals, that mutations in glial cells can produce epileptic seizures. Counteracting the effects of the glial mutation may be a promising new strategy for developing epilepsy treatments, says Troy Littleton, an MIT professor of biology and leader of the research team.
“This signaling pathway from glia to neurons likely controls the firing properties of neurons, and if it’s hyperactivated, the neurons fire too much. Anything that would prevent that hyperactivity might be a potential therapeutic,” says Littleton, who is also a member of MIT’s Picower Institute for Learning and Memory.
Littleton and lead author Jan Melom, a graduate student in MIT’s Department of Biology, described their new findings in the Jan. 16 online edition of the Journal for Neuroscience.
Origin of seizures
Glial cells, which make up about half of the cells in the brain, have many supporting roles, including cushioning neurons and helping them form connections with one another. However, in recent years, neuroscientists have found evidence of more important functions, such as assisting in communication between neurons.
Previous studies had shown that glial cells become hyperactive during epileptic seizures, but it wasn’t clear whether the glial cells’ hyperactivity was causing the seizure or if they were merely reacting to neuronal hyperactivity. The new study shows that the glial cells are indeed provoking the seizure, according to the researchers.
“We know that we’re knocking out a gene that’s only expressed in this one cell population, of glia,” Melom says. “It’s unambiguous that the seizure originally arises from defects in the glia rather than in the neurons.”
Littleton’s lab uses fruit flies as a model organism for studies of neuronal development and the formation of synapses, the connections between neurons. To pinpoint the genes involved in these processes, they mutate fruit fly genomes and screen the resulting flies for abnormal traits. Most of the mutations occur in neurons, but in this case, the researchers found that flies with glial mutations in a gene they named zydeco caused the flies to undergo seizures at high temperatures.
Further study of the zydeco gene revealed that its normal function is to get rid of calcium inside the glia. This helps to regulate glial fluctuations in calcium levels, which the MIT team observed for the first time in fly glia.
When zydeco is mutated, the fluctuations stop, causing calcium to build up inside the glial cells. Through an unknown process, this induces nearby neurons to become hyperactive and makes them more likely to seize in response to heat, cold or shaking. “It’s clear that their nervous systems are hyperexcitable to a lot of environmental stimuli,” Littleton says.
This is one of the few examples scientists have found of glia activity influencing neurons’ firing.
“Neurons are still carrying the main information,” Littleton says. “They’re the ones talking. But glia are somehow listening in and modulating the neurons’ output. If that output is disrupted by glia, it causes the whole system to fail.”
Sources of excitation
The researchers are now exploring the link between glial calcium levels and neuronal excitability. One theory is that too much calcium produces a flood of neurotransmitters or other factors that glia normally secrete in a tightly regulated fashion. This unregulated secretion could hyperexcite the neurons, Littleton says.
“Glia are enlisted into the conversation that neurons are having, and they may modulate that by releasing these factors that control activity,” Littleton says. “Elevated calcium may create a situation where the glia are no longer releasing these factors in a regulated way, but they’re releasing them all the time.”
In the human brain, astrocytes are the cells most similar to the fruit fly glial cells observed in this study, according to the researchers. These cells envelop the cell bodies of neurons and are also the most abundant type of glial cell in the human brain. It is not yet known whether these cells express one of the five human versions of the zydeco gene.
Although further study is needed, the findings suggest that preventing calcium from building up in glial cells or astrocytes could reduce neuronal excitability and decrease the tendency to seize in response to environmental stimuli, according to the researchers. “It’s still a long way off, but the fly work suggests that reducing glial calcium levels would be effective in toning down excitability,” Littleton says.
Such drugs might also eliminate the sedative side effects often seen with current epilepsy medications, says Maiken Nedergaard, a professor of neurosurgery at the University of Rochester who was not involved in the research.
“You could envision new medications that target glial cells and don’t slow down the brain as much,” Nedergaard says, adding that the study is a major step forward in understanding glial cells’ role in the brain.
The MIT researchers are now looking for other components of the pathway that connects glial calcium levels to neuronal hyperactivity, which could lead to the discovery of other potential drug targets.
The study was funded by the National Institutes of Health.

